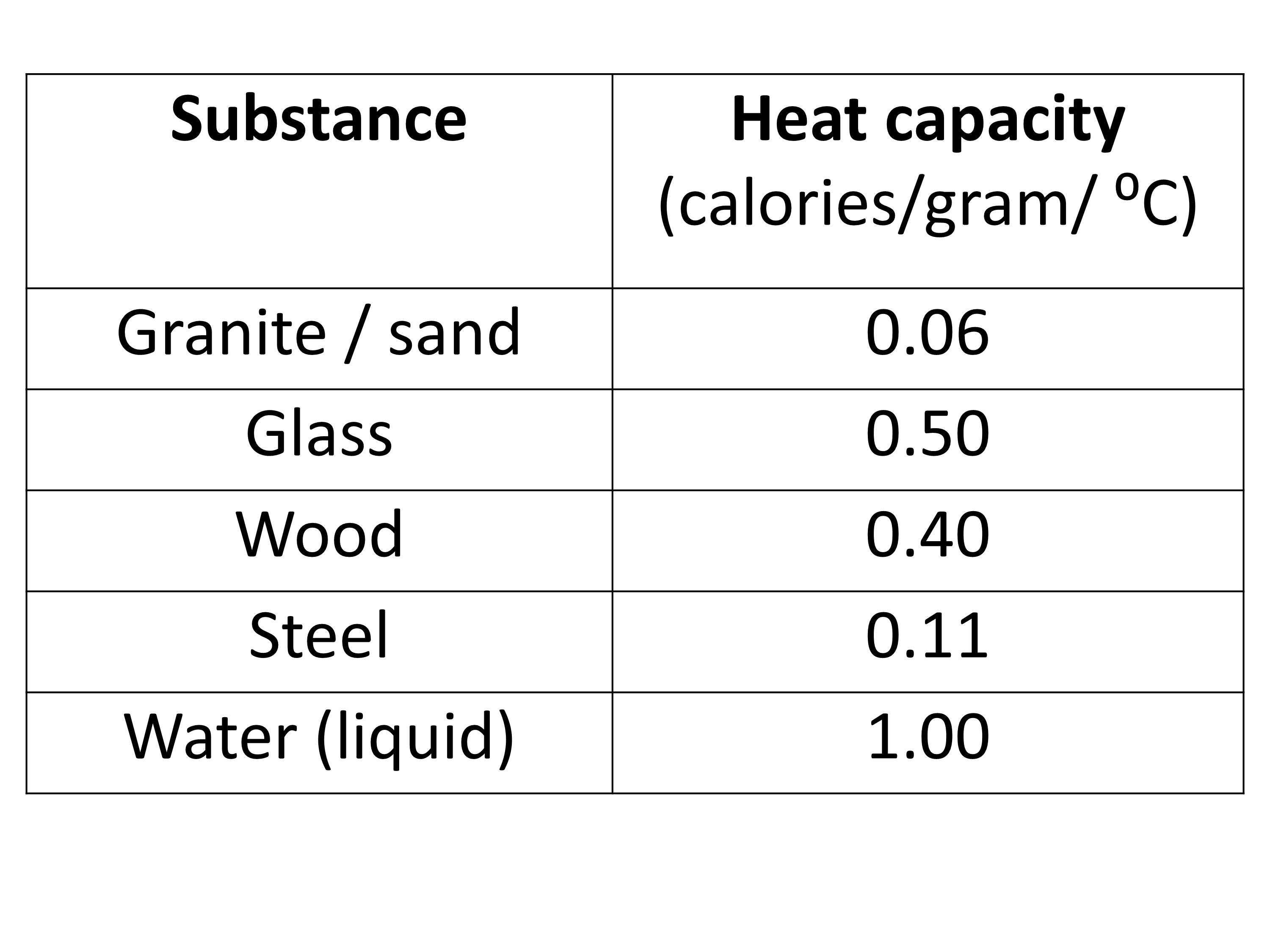
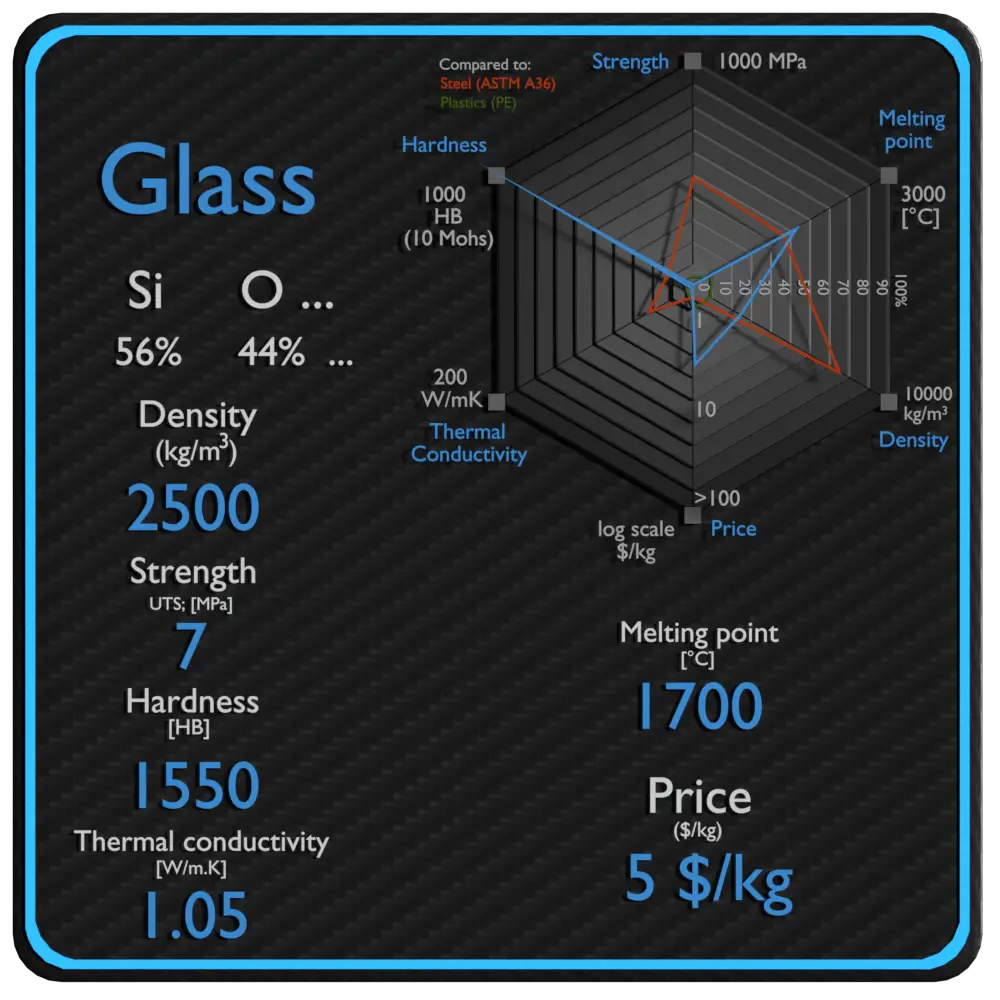
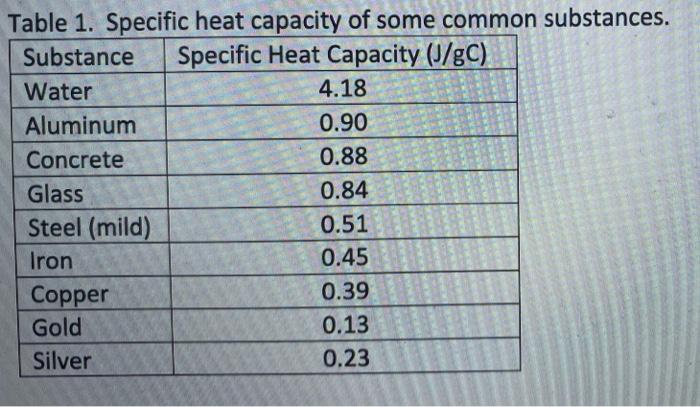
Table 2 from High-temperature heat capacities of corundum , periclase , anorthite , Q 1 { lrSirO , glass ,-muscovite , pyrophtllite , KAISisOB glass , grossular ' and NaAlSirOr glass | Semantic Scholar

Specific heat capacities for an as-deposited thin toluene film (solid... | Download Scientific Diagram

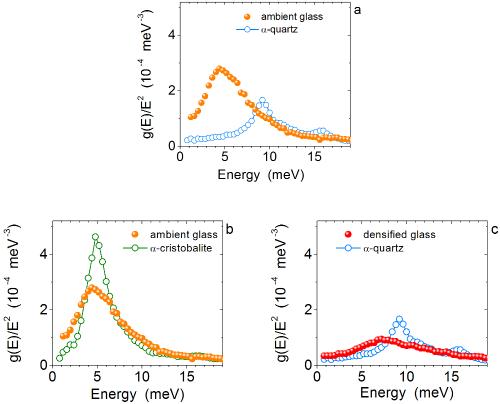
Specific heat and magnetization studies of spin-glass like transition in nanogranular Cu90Co10 ribbon - ScienceDirect

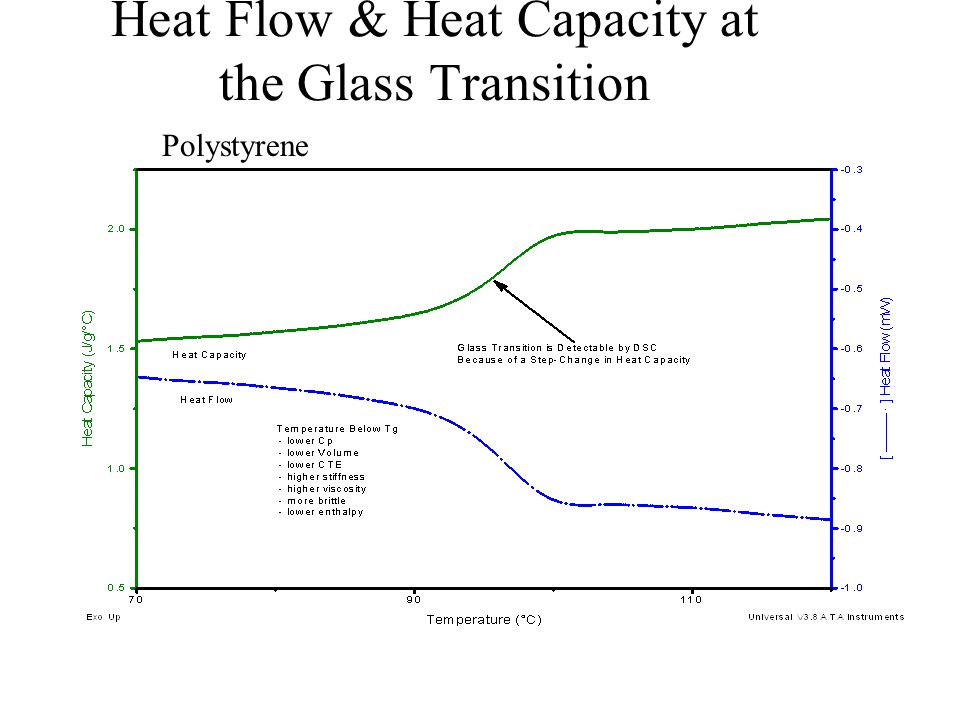
Heat capacity measurements and modeling of polystyrene glass transition in a wide range of cooling rates - ScienceDirect
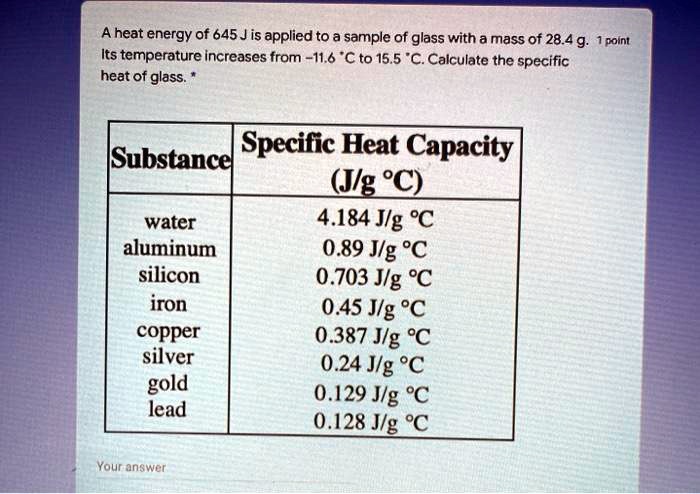
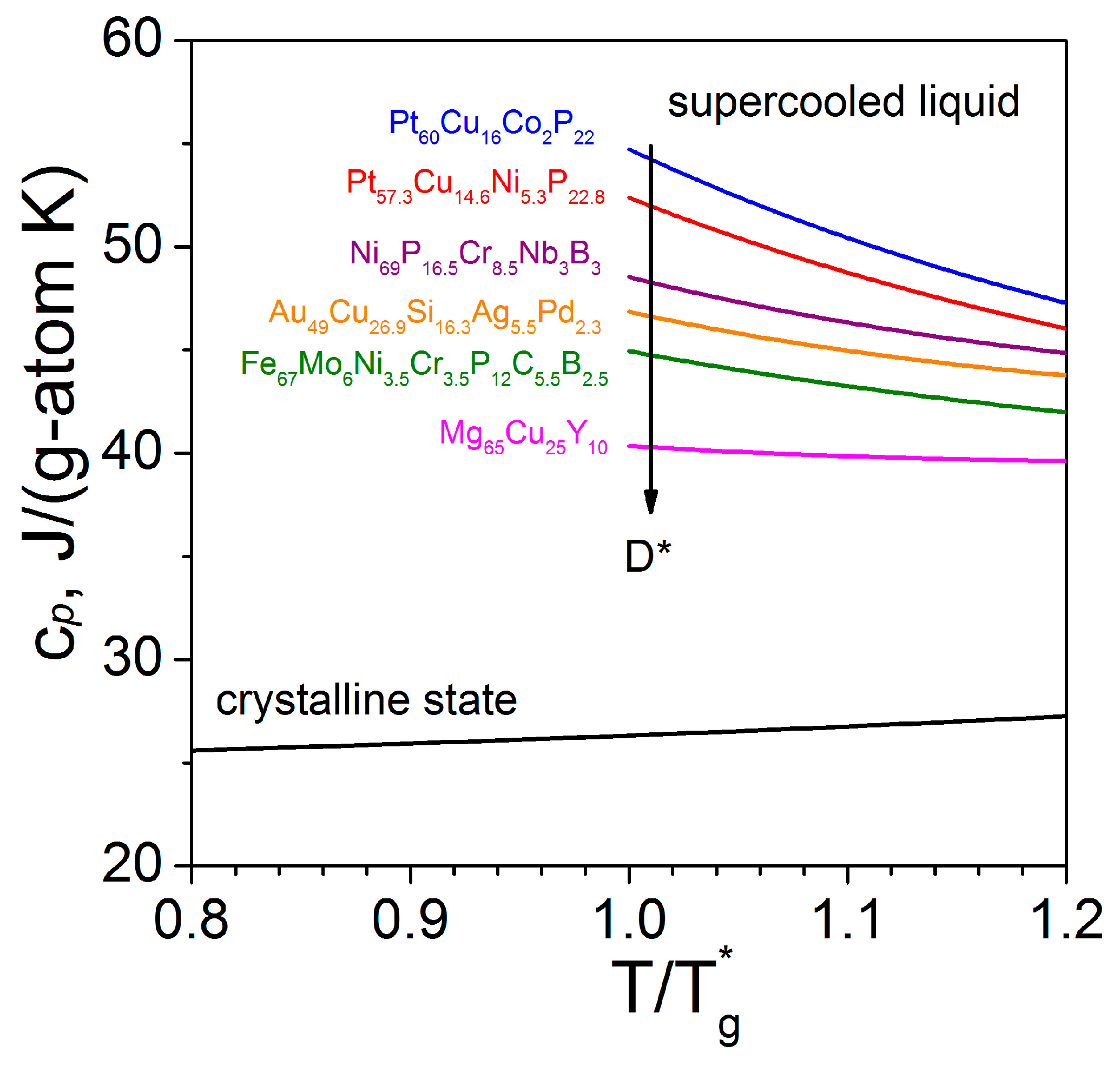
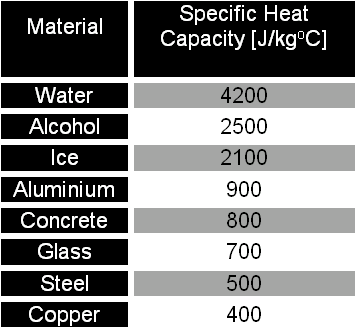
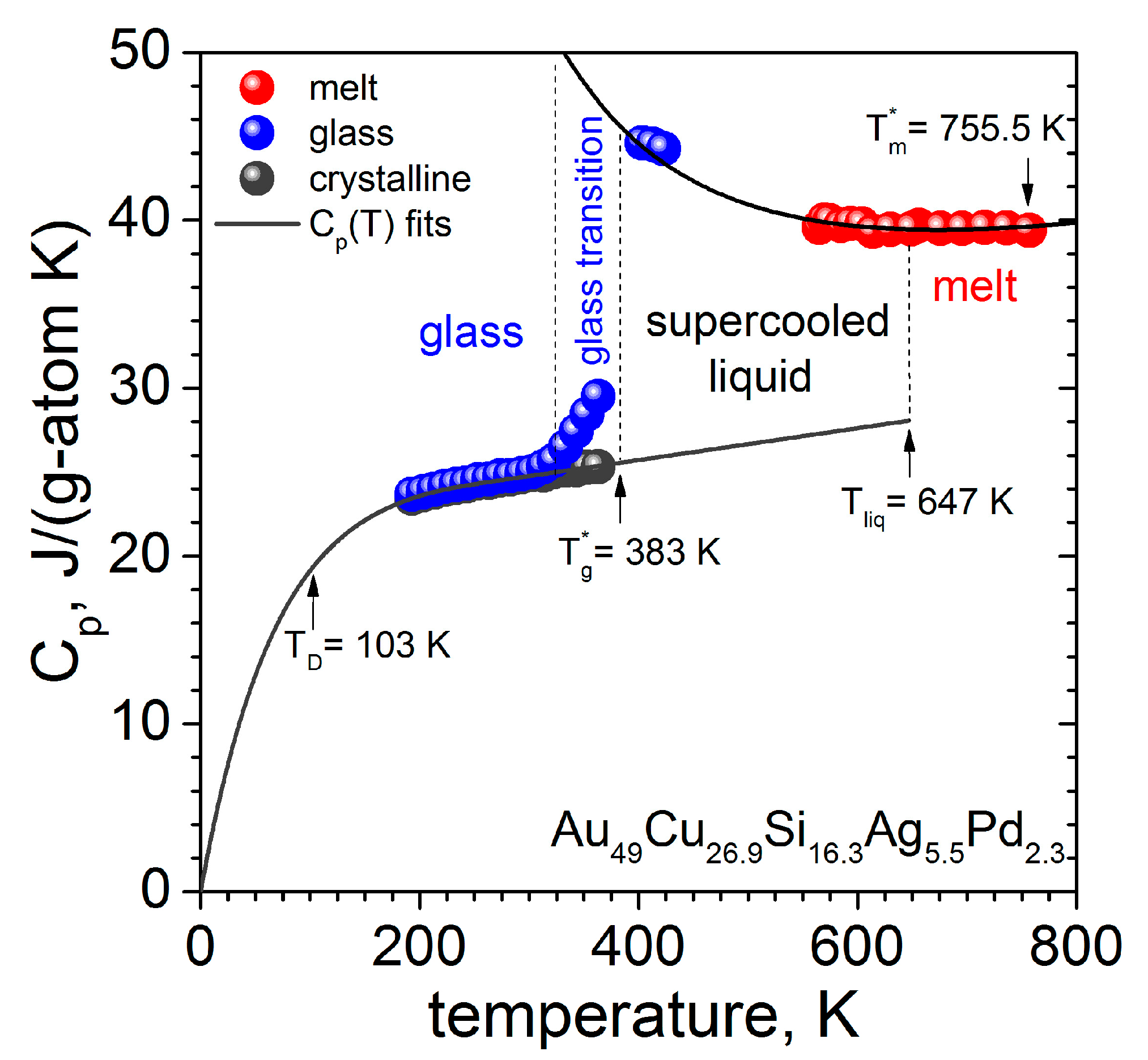



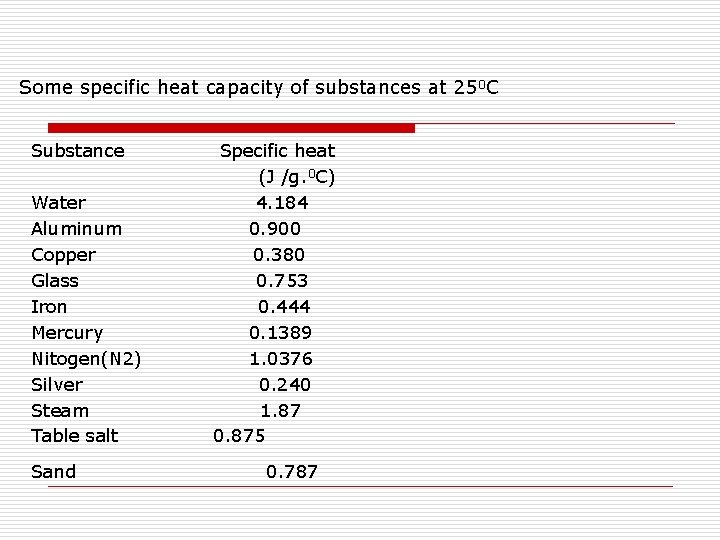
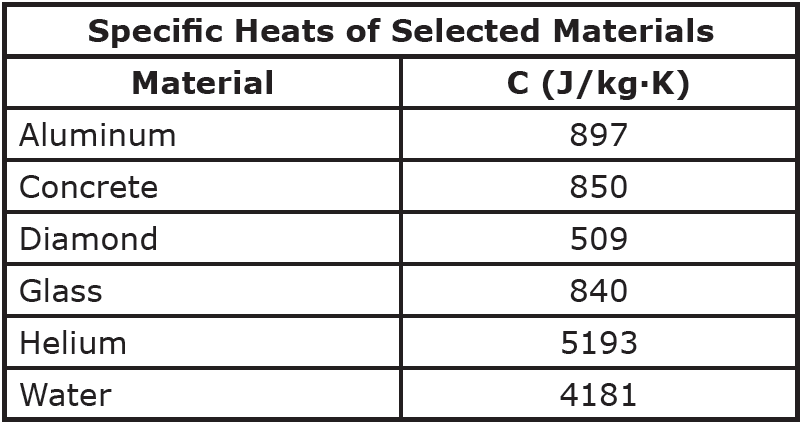
.jpg)